Research and CV_
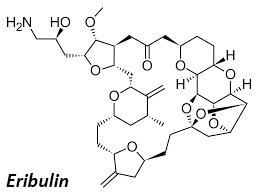
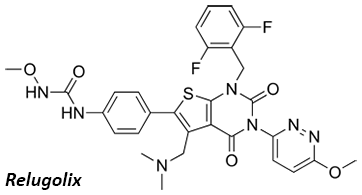
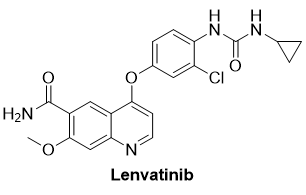
Ubin has a broad interest in all aspects of organic chemistry, with expertise spanning total synthesis, catalysis, process development, and medicinal chemistry. Ubin has contributed to the field through book chapters, review articles, patents, and original research publications. With experience in both academic and industrial settings, Ubin has led process and medicinal chemistry teams, working on the development of Kinase inhibitors and complex APIs such as Eribulin, Relugolix, and Lenvatinib. Most recently, Ubin transitioned to a role at MFC, where he oversees CMO/CDMO projects ranging from process chemistry to early-stage drug discovery.
![]() Birth details: South Korea (1986), 38 years old.
Birth details: South Korea (1986), 38 years old.
![]() Nationality: New Zealand
Nationality: New Zealand
![]() E-mail: ubinkim@gmail.com
E-mail: ubinkim@gmail.com
![]() Language: English (fluent, first language); Korean (oral, fluent)
Language: English (fluent, first language); Korean (oral, fluent)
![]() 2024 - current: Principal Research Engineer (수석연구원) / Global business development
2024 - current: Principal Research Engineer (수석연구원) / Global business development
Gwacheon, South Korea
![]() 2020 - 2024: Senior Researcher (책임연구원) / Project Leader / Team Leader
2020 - 2024: Senior Researcher (책임연구원) / Project Leader / Team Leader
Suwon, South Korea
![]() 2019 - 2020: Senior Researcher (선임연구원) / Project Leader / Team Leader
2019 - 2020: Senior Researcher (선임연구원) / Project Leader / Team Leader
Songdo, Incheon, South Korea Collapse
![]() 2015-2018: Postdoctoral Associate
2015-2018: Postdoctoral Associate
Advisor: Professor Sang-gi Lee
Seoul, South Korea
![]() 2009-2014: Ph. D. (download PhD thesis here)
2009-2014: Ph. D. (download PhD thesis here)
Advisor: Professor Margaret A. Brimble
Auckland, New Zealand
![]() 2008: BSc (Honours) 1st Class. (download BSc Hons. thesis here)
2008: BSc (Honours) 1st Class. (download BSc Hons. thesis here)
Advisor: Professor Margaret A. Brimble
Collapse
Industry
Method for preparing relugolix.
Kim, U. B.; Saidhareddy, P.; Yu, H.; Myeong, I.-S.; Lee, S. J.; Lee, K. Y.; Oh, C.
[Patent filed] WO2025127790A1. December, 10, 2024 | [pdf] | [preview]
Process Chemistry
Proprietary Synthesis of the C27-C35 Fragment of Eribulin and it’s Elaboration Towards the C14-C35 Sub-unit.
Kim, U B.; Samala, S.; Jung, J.; Song, J.; Lee, K-Y.; Oh, C-Y.; Shin, H. Org. Process Res. Dev., 2024, 28, 2832–2843.
DOI: acs.oprd.4c00150 | [pdf] | [preview]
Process Chemistry
Scalable synthesis of the C14-C23 fragment of Eribulin and Halichondrin B.
Kim, U B.1; Samala, S.1; Kim, N.; Bogonda, G.; Lago-Santome, H.; Jeong, Y.; Kim, J.; Jung, J.; Jeon, S-H.; Lee, S. J.; Shin, H. Bull. Korean. Chem. Soc., 2022, 43, 1367. (1 = Equal contribution)
DOI: 10.1002/bkcs.12624 | [pdf] | [preview]
Process Chemistry
Synthesis of the C1–C13 Fragment of Eribulin on a Kilogram Scale.
Kim, S. T.; Park, Y.; Kim, N.; Gu, J.; Son, W.; Hur, J.; Lee, K.; Baek, A.; Song, J. Y.; Kim, U B.; Lee, K-Y.; Oh, C-Y.; Park, S.; Shin, H. Org. Process. Res. Dev., 2022, 26, 123.
DOI: 10.1021/acs.oprd.1c00295 | [pdf] | [preview]
Process Chemistry
Process Development of Tacalcitol.
Lee, S. J.; Moon, H. W.; Lee, K-Y.; Oh, C. Y.; Kim, U B.; Shin, H. Org. Process. Res. Dev., 2021, 25, 982.
DOI: 10.1021/acs.oprd.1c00010 | [pdf] | [preview]
Medicinal Chemistry
Heteroaryl derivatives, and it’s pharmaceutical composition for use in preventing cancer containing the same as an active ingredient.
Kim, U B.; Lee, Y. H.; Kang, S-i, Hwnag, S. A.; Kim, D. M.; Kim, S. S.; Jung, M. H.; Kim, H. K.; Jung, H. R.; Kim, Y. S.; Jang, H. J.; Choi, J. E.; Lee, S. H.; Son, J. B., Kim, N. D.
[Patent filed] PCT/KR2020/003558. March, 13, 2020 | [pdf] | [preview]
Synergistic Dual Transition Metal Catalysis.
Kim, U B.; Jung, D. J.; Jeon, H. J.; Rathwell, K.; Lee, S.-g. Chem. Rev., 2020, 120, 13382-13433.
DOI: 10.1021/acs.chemrev.0c00245 | [pdf] | [preview]
Catalysis, Book Chapter
Rhodium/Palladium Dual Catalysis.
Kim, U B.; Lee, S.-g. Science of Synthesis. 2019, 1, 57
[Book Chapter, Ed.: Molander, G. A.] DOI: 10.1055/sos-SD-231-00029
Catalysis
Palladium-Catalyzed Divergent Cyclopropanation by Regioselective Solvent-Driven C(sp3)-H Bond Activation.
Chung, D. S.; Lee, J. S.; Ryu, H.; Park, J.; Kim, H.; Lee, J. H., Kim, U B..; Lee, W. K.; Baik, M.-H.*; Lee, S.-g.
Angew. Chem. Int. Ed., 2018, 57, 15460-15464.
DOI: 10.1002/anie.201809133 | [pdf] | [preview]
Catalysis
Rh(II)/Mg(OtBu)2‑Catalyzed Tandem One-Pot Synthesis of 1,4-Oxazepines and 1,4-Oxazines from N‑Sulfonyl-1,2,3-triazoles and Glycidols.
Ko, Y. O.; Jeon, H. J.; Jung, D. J.; Kim, U B.; Lee, S.-g. Org. Lett., 2016, 18, 6432-6435.
DOI: 10.1021/acs.orglett.6b03328 | [pdf] | [preview]
Methodology
Synthesis of ketones via organolithium addition to acid chloride using continuous flow chemistry.
Moon, S-Y.; Jung, S-H.; Kim, U B.; Kim, W-S. RSC Adv., 2015, 5, 79385-79390.
DOI: 10.1039/C5RA14890A | [pdf] | [preview]
Methodology
A Synthetic Approach to N-Aryl Carbamates via Copper-Catalyzed Chan–Lam Coupling at Room Temperature.
Moon, S-Y.1; Kim, U B.1; Sung, D.-B.; Kim, W-S. J. Org. Chem., 2015, 80(3), 1856–1865. (1 = Equal contribution)
DOI: 10.1021/jo502828r | [pdf] | [preview]
Total Synthesis of Chaetoquadrins H and I.
Kim, U B.; Dalebrook, A. F.; Furkert, D. P.; Brimble, M. A. Synlett. 2013, 24(6), 723-726.
DOI: 10.1055/s-0032-1318333 | [pdf] | [preview]
Total Synthesis
Total synthesis of Chaetoquadrins A–C.
Kim, U B.; Furkert, D. P.; Brimble, M. A. Org. Lett., 2013, 15, 658–661.
DOI: 10.1021/ol303482k | [pdf] | [preview]
Total Synthesis
Synthesis of (±) Wine Lactone and Its Analogues by a Diels–Alder Approach.
O'Connor, P. D.; Kim, U B.; Brimble, M. A. Eur. J. Org. Chem. 2009, 4405–4411.
DOI: 10.1002/ejoc.200900486 | [pdf] | [preview]
Collapse